Localities
At the Woods mine, New South Wales, Australia, hausmannite forms by oxidation and loss of SiO2 from neotocite and tephroite. (AJM 15.1&2.15)
At the Xianghualing Mine, Xianghualing Sn-polymetallic ore field, Linwu County, Chenzhou, Hunan, China, black hausmannite is found with with galena, pyrite, chalcopyrite and bornite (AESS).
Hausmannite from Xianghualing - Image
At the type locality, Oehrenstock, Langewiesen, Ilmenau, Ilm-Kreis, Thuringia, Germany, hausmannite occurs in a manganese ore deposit.
Hausmannite from Oehrenstock - Image
At Ilfeld, Harztor, Nordhausen, Thuringia, Germany, hausmannite pseudomorphs after manganite have been found with minor baryte (KL p147).
Hausmannite from Ilfeld - Image
At the Wyndham pit, Bigrigg, Egremont, Copeland, Cumbria, England, UK, hausmannite is associated with hematite (SY p162).
Alteration
braunite to hausmannite, SiO2 and O2
3Mn2+Mn3+6O8(SiO4) ⇌ 7Mn2+Mn3+2O4 +3SiO2 + O2
(AM80.565)
braunite to hausmannite, rhodonite and O2
2Mn2+Mn3+6O8(SiO4) ⇌ 4Mn2+Mn3+2O4 + 2Mn2+SiO3 + O2
A higher temperature favours the forward reaction
(AM80.565).
braunite to tephroite, hausmannite and O2
3Mn2+Mn3+6O8(SiO4) ⇌ 3Mn2+2(SiO4) + 5Mn2+Mn3+2O4 + 2O2
(AM80.571)
hausmannite and O2 to bixbyite-(Mn)
4Mn2+Mn3+2O4 + O2 ⇌ 6Mn2O3
(AM80.560-575)
hausmannite and quartz to rhodonite and O2
2Mn2+Mn3+2O4 + 6SiO2 ⇌ 6Mn2+SiO3 + O2
(AM80.571)
hausmannite and rhodonite to tephroite and O2
2Mn2+Mn3+2O4 + 6Mn2+SiO3 ⇌ 6Mn2+2SiO4 + O2
A higher temperature favours the forward reaction (AM80.565)
manganosite and O2 to hausmannite
6MnO + O2 ⇌ 2Mn2+Mn3+2O4
A higher temperature favours the forward reaction
(AM80.571)
rhodochrosite and O2 to hausmannite and CO2
6MnCO3 + O2 ⇌ 2Mn2+Mn3+2O4 +6CO2
A higher temperature favours the forward reaction (AM80.571)
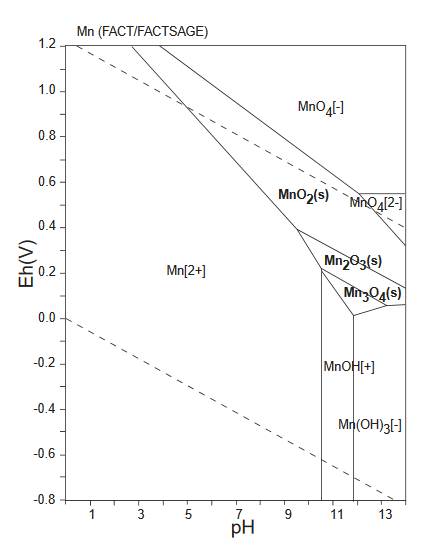
The diagram below is a Pourbaix diagram for manganese (GSJ). It shows the relationship between akhtenskite MnO2, bixbyite-(Mn) Mn2O3 and hausmannite Mn3O4.
Back to Minerals