It is found in plutonic igneous environments as an accessory mineral in feldspar-rich igneous rocks such as granite, and in pegmatites and carbonatites.
Large ore bodies of hematite are usually of sedimentary origin. Hematite is also found in red sandstone as the cementing material that binds the quartz grains together.
Hematite occurs both in contact and regional metamorphic deposits, where it may have originated from the oxidation of limonite, siderite or magnetite.
It occurs in disseminated hydrothermal replacement deposits and in hydrothermal replacement lodes, as well as in the oxidation zone of epithermal (low temperature) and mesothermal (moderate temperature) hydrothermal veins.
It may also occur as a sublimation due to volcanic activity.
Hematite is a common constituent of marl.
Localities
At the Payún Matrú volcano, Altiplano de Payún Matrú, Agua Escondida District, Malargüe Department, Mendoza Province, Argentina, hematite pseudomorphs after magnetite have been found (KL p138).
Hematite from the Payún Matrú volcano - Image
The Two Mile and Three Mile deposits, Paddy's River, Paddys River District, Australian Capital Territory, Australia, are skarn deposits at the contact between granodiorite and volcanic rocks. Hematite is a primary oxide that occurs in granular veins and intergrowths with magnetite and rarely as compact masses of large blades in quartz (AJM 22.1.38).
At the Mount Kelly deposit, Gunpowder District, Queensland, Australia, the copper ores overlie primary zone mineralisation consisting of quartz-dolomite-sulphide veins hosted in siltstone and schist. Hematite occurs as a red groundmass or as coatings on fractures associated with goethite, as inclusions in quartz, surrounding pyrite and associated with brochantite (AJM 22.1.21).
At the Blue Point mine and at the Thunder Bay Amethyst mine, Thunder Bay, Ontario, Canada, microscopic spherulites of hematite occur as inclusions in quartz variety amethyst, often imparting a characteristic red coloration (R&M 94.4.320 and 332-333).
Hematite from the Blue Point Mine - Image
At Jinlong Hill, Longchuan Co. (Lungchuan Co.), Heyuan, Guangdong, China, clusters of blackish, tabular hematite crystals occur scattered among colourless prismatic quartz crystals (AESS).
Hematite from Jinlong - Image
In the White Desert on the border between Egypt and Libya hematite pseudomorphs after marcasite have been found (KL p139).
At Johanngeorgenstadt, Erzgebirgskreis, Saxony, Germany, hematite was the most common and important Of the ores extracted; it occurred in many varieties of red ore mixed with varying amounts of earth and clay. Fibrous masses of “blood stone” with reniform surfaces and shell-like structures, as well as stalactiform shapes, were very common. Radial slabs of hematite of this type reaching 1 metre in length came from the Hilfe Gottes mine. There were also pseudomorphs of red iron oxide after calcite, less commonly after baryte, anhydrite and fluorite. Specular hematite and a porous hematite with small, scaly, transparent red crystals were present in minor amounts in the iron ore veins.
In the bismuth - cobalt - uranium ore veins which were mined from 1945 to 1958, specular hematite was found in various spherical and tabular habits near areas of pitchblende in dolomite-ankerite or calcite gangue. Small, scaly, red to reddish brown crystals were seen widely dispersed over faces of calcite and fluorite crystals, or over “coxcomb” formations of quartz (MinRec 55.5.598-599).
At the Monte Cervandone area, Devero Alp, Baceno, Verbano-Cusio-Ossola Province, Piedmont, Italy, “Iron rose” aggregates of tabular hematite crystals to 4 cm occur widely in the orthogneiss of the Monte Leone nappe; significant finds also have been made in the Chummibort, Ritter Pass, Chriegalptal, Wannigletscher and Schinhorn areas. The paragneiss of the Lärcheltini Zone has produced isolated tabular crystals of hematite to 4 cm, with oriented crystals of rutile and/or anatase (MinRec 56.3.317-318).
Hematite from the Monte Cervandone area - Image
At Charcas, Charcas Municipality, San Luis Potosí, Mexico, the primary minerals are sphalerite, galena, chalcopyrite, bornite, tetrahedrite, arsenopyrite, pyrite and silver minerals such as jalpaite, diaphorite and acanthite. In the host rock, as metamorphic or alteration minerals, danburite, datolite, hedenbergite, epidote, chlorite, andradite, actinolite and wollastonite have been reported.
Quartz, calcite and danburite crystallised during the entire life of the systems, throughout the intrusive emplacement, metamorphism, and mineralising events. With depth, both sphalerite and galena decrease while chalcopyrite increases.
Secondary sulphides formed include bornite, covellite, digenite and chalcocite. Native silver, native gold, hematite and goethite were deposited after the sulphides (Minrec 55.6.727-728).
Hematite shows a colloform morphology as a secondary mineral in the oxidation zones along the metamorphic aureole and in small veins, and is associated with wulfenite. In the deeper, non-oxidised environment, the crystals form lamellae 2 to 4 mm in size. These generally occur in the skarn zones, associated with andradite, calcite and quartz (Minrec 55.6.757).
At Vordere Chollergrabe, Lärchultini, Binn, Goms, Valais, Switzerland, a spectacular specimen of hematite has been found (Mindat photo).
Hematite from Vordere Chollergrabe - Image
At Croft Quarry, Croft, Blaby, Leicestershire, England, UK, there appear to be at least four generations of hematite. It occurs as inclusions in analcime, as coatings on analcime and on analcime epimorphs after laumontite, and associated with or coating calcite (JRS 20.17).
At the Llynclys quarry, near Oswestry, Shropshire, England, UK, hematite occurs on dolomite (RES p294).
At Coed-y-Brenin deposit, Ganllwyd, Gwynedd, Wales, UK, magnetite forms scattered crystals to 1 mm in size, associated with isolated specular hematite rosettes to 2.5 mm, both phases occurring embedded in or perched on chlorite (JRS 21.115).
At the Magma mine, Pioneer District, Pinal county, Arizona, USA, hematite is the most common gangue mineral, and crystals have been found to 2.5 cm, some with a dusting of malachite, in association with calcite crystals (R&M 95.1.86).
Hematite from the Magma Mine - Image
At the PC Mine, Cataract Mining District, Jefferson county, Montana, USA, hematite was found primarily as shiny platelets to 2 mm across included in quartz (R&M 96.6.494).
At the Dafoe property, Pierrepont, St. Lawrence county, New York, USA, hematite occurs as coatings, sometimes thick, and massive fillings in almost all areas of the property and on all specimens found there. It is present as reddish-brown staining in and on calcite and quartz crystals. Occasionally it is found as attractive red phantoms in clear quartz. Other forms sometimes encountered are red to brown spherical masses and splendent, bladed crystals to 1 cm. These are associated with calcite and quartz crystals and in rare cases are found as attractive coatings on selective faces of calcite crystals. Hematite rarely forms spectacular pseudomorphs after large, complex, octahedral magnetite crystals (R&M 97.3.247-248).
Alteration
Hematite may form as an alteration product of ilmenite (AJM 18.2.26).
aegirine, epidote and CO2 to albite, hematite, quartz, calcite and H2O
4NaFe3+Si2O6 + 2Ca2(Al2Fe3+ [Si2O7](SiO4)O(OH) + 4CO2 → 4Na(AlSi3O8) + 3Fe2O3 + 2SiO2 + 4CaCO3 + H2O
(DHZ 2A p511)
calcite, hematite and quartz to andradite and CO2
3CaCO3 + Fe2O3 + 3SiO2 → Ca3Fe3+2Si3O12 + 3CO2
fayalite, oxygen and H2O to hematite and silicic acid
2Fe2SiO4 + O2 + 4H2O → 2Fe2O3 + 2H4SiO4
On prolonged exposure to the air Fe2+ compounds are oxidised to Fe3+ compounds according to reactions such as the one above (KB p334).
hematite and H2O to goethite
Fe2O3 + H2O ⇌ 2FeO(OH)
Both forward and reverse reactions are slow, but equilibrium in most natural environments is displaced to the left, favouring the formation of hematite (KB p362).
hematite, wüstite, quartz and calcite to andradite, hedenbergite, magnetite and CO2
2Fe2O3 + 2FeO + 5SiO2 + 4CaCO3 → Ca3Fe3+2(SiO4)3 + CaFe2+Si2O6 +Fe2+Fe3+2O4 +4CO2
magnetite to hematite
2Fe3O4 + ½O2 ⇌ 3Fe2O3
Equilibrium is to one side or the other depending on temperature and pressure.
siderite, oxygen and H2O to hematite and silicic acid
2Fe2CO3 + O2 + 4H2O → 2Fe2O3 + 2H2CO3
On prolonged exposure to the air Fe2+ compounds are oxidised to Fe3+ compounds according to reactions such as the one above (KB p334).
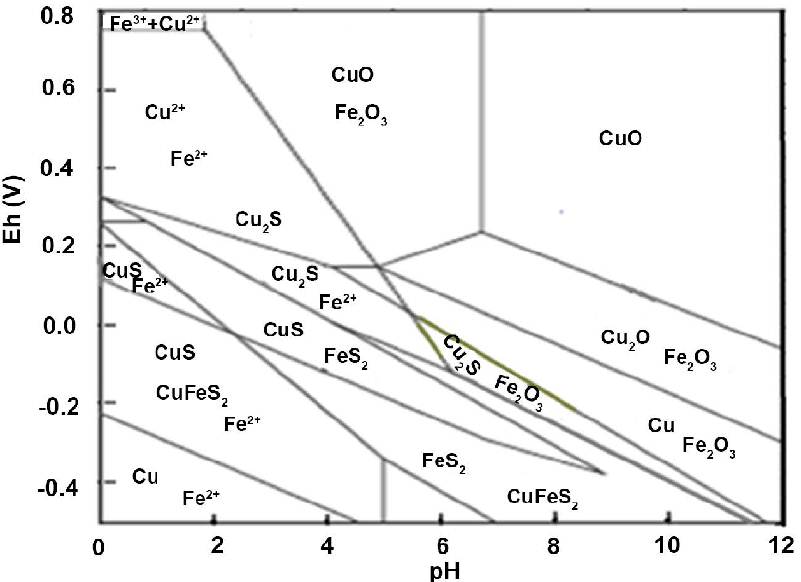
The diagram below is a Pourbaix diagram for Cu-Fe-S-H2O (IJNM 07(02).9.23). It shows the relationship between copper Cu, chalcopyrite CuFeS2, tenorite CuO, covellite CuS, cuprite Cu2O, chalcocite Cu2S, pyrite FeS2 and hematite Fe2O3.
Back to Minerals