The variety viridine typically occurs in low-grade regionally metamorphosed manganese-rich rocks, associated with piemontite, spessartine, chlorite and hematite (Lauf p183).
Andalusite occurs in granite, gneiss, phyllite and schist.
It is a mineral of the albite-epidote-hornfels, hornblende-hornfels, pyroxene-hornfels, amphibolite and granulite facies.
Localities
Santa Teresa, Espírito Santo, Brazil, is the only locality in the Espírito Santo Pegmatite District where andalusite occurs, in some colluvial deposits near the town of Santa Teresa. The crystals, between 1 and 2 cm long, are well preserved, indicating that they have not traveled far from their source rocks, which probably were the schists and gneisses that host the pegmatites.
The andalusites from this mine are considered the best in Brazil. The crystals, found in metamorphic rocks rather than pegmatites, are tetragonal prisms with flat basal terminations, from 2 to 5 cm long, predominantly olive-green, though some are pale to dark brown, with red internal reflections; they are dichroic, appearing reddish when viewed down their length and olive-green in perpendicular directions (Minrec 54.703).
In Xinjiang, China, andalusite has been found as dull whitish crystals up to 1 cm long embedded in a grey matrix (AESS).
Andalusite from Xinjiang - Image
At the type locality, El Cardoso de la Sierra, Guadalajara, Castile-La Mancha, Spain, andalusite occurs in low-pressure and low-temperature metamorphic rocks (Mindat).
Alteration
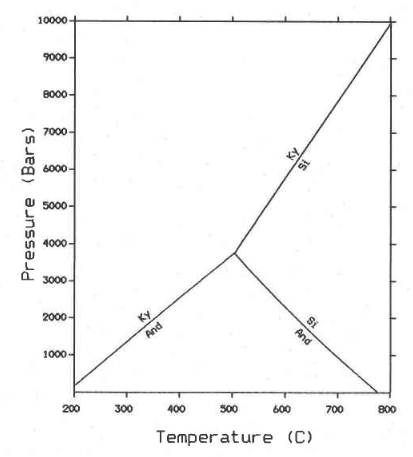 Andalusite, sillimanite and kyanite
are paramorphs; they are in equilibrium at a
pressure of 3.75 kbar and temperature 504oC
(amphibolite facies)
(AM78.298-315).
Andalusite, sillimanite and kyanite
are paramorphs; they are in equilibrium at a
pressure of 3.75 kbar and temperature 504oC
(amphibolite facies)
(AM78.298-315).
Andalusite is unstable at high pressure and cannot form at pressure above 3.75 kbar.
augite and andalusite to enstatite- ferrosilite and anorthite
Ca(Fe,Mg)Si2O6 +Al2SiO5 → (Mg,Fe2+)SiO3 + Ca(Al2Si2O8) (DHZ 2A p126).
chloritoid and andalusite to staurolite and quartz and H2O
4Fe2+Al2O(SiO4)(OH)2 + 5Al2OSiO4 ⇌ 22Fe2+2Al9Si4O23(OH) + SiO2 + 3H2O
Increasing temperature favours the forward reaction. At higher pressure kyanite replaces andalusite in the above reaction (AM61.699-709).
enstatite-ferrosilite and andalusite to Fe rich cordierite and spinel- hercynite
5(Mg,Fe2+)SiO3 + 5 Al2SiO5 → 2(Mg,Fe2+)2Al4Si5O18 + (Mg,Fe2+)Al2O4
In medium-grade thermally metamorphosed argillaceous rocks originally rich in chlorite and with a low calcium content, in an SiO2 deficient environment the association of andalusite with enstatite- ferrosilite is excluded by the above reaction (DHZ 2A p134).
enstatite-ferrosilite, andalusite and quartz to Fe-rich cordierite
2(Mg,Fe2+)SiO3 + 2Al2SiO5 + SiO2 → (Mg,Fe2+)2Al4Si5O18
In medium-grade thermally metamorphosed argillaceous rocks originally rich in chlorite and with a low calcium content, the association of andalusite with enstatite- ferrosilite is excluded by the above reaction (DHZ 2A p134).
kaolinite to andalusite, pyrophyllite and H2O
3Al2Si2O5(OH)4 ⇌ 2Al2OSiO4 + Al2Si4O10(OH)2 + 5H2O
At 1 kbar pressure the equilibrium temperature for the reaction is about 320oC (albite-epidote-hornfels facies), with the equilibrium to the right at higher temperatures and to the left at lower temperatures. (SERC, AM61.699-709)
kaolinite and diaspore to andalusite and H2O
Al2Si2O5(OH)4 + 2AlO(OH) ⇌ 2Al2OSiO4 + 3H2O
At 1 kbar pressure the equilibrium temperature for the reaction is about 320oC (albite-epidote-hornfels facies), with the equilibrium to the right at higher temperatures and to the left at lower temperatures (SERC, AM61.699-709).
margarite and quartz to anorthite, andalusite and H2O
CaAl2(Al2Si2O10)(OH)2 + SiO2 ⇌ Ca(Al2Si2O8) + Al2OSiO4 + H2O
The equilibrium temperature for this reaction at 2 kbar pressure is about 440oC (greenschist facies), with the equilibrium to the right at higher temperatures, and to the left at lower temperatures (SERC, AM61.699-709).
paragonite and quartz to albite and andalusite and H2O
NaAl2(Si3Al)O10(OH)2 + SiO2 ⇌ Na(AlSi3O8) + Al2OSiO4 + H2O
Increasing temperature favours the forward reaction (AM61.699-709).
pyrophyllite to andalusite, quartz and H2O
Al2Si4O10(OH)2 ⇌ Al2SiO5 + 3SiO2 + H2O
The equilibrium temperature for this reaction at 1.8 kbar pressure is 414oC (greenschist facies), with the equilibrium to the right at higher temperatures, and to the left at lower temperatures (JVW p539).
pyrophyllite and diaspore to andalusite and H2O
Al2Si4O10(OH)2 + 6AlO(OH) ⇌ 4Al2SiO5 + 4H2O (JVW p 553)
This reacton is a low pressure reaction, occurring below about 1.9 kbar. Increasing temperature favours the forward reaction (SERC).
Back to Minerals