Localities
The Two Mile and Three Mile deposits, Paddy's River, Paddys River District, Australian Capital Territory, Australia, are skarn deposits at the contact between granodiorite and volcanic rocks. antlerite is a secondary sulphate that occurs with brochantite in sulphide-bearning magnetite skarn (AJM 22.1.43).
At Caldbeck Fells, Cumbria, England, UK, antlerite is very rare. It has been found at the Old Potts Gill mine as powdery aggregates in malachite (C&S).
At Parys Mountain, Anglesey, Wales, UK, oxidation of chalcopyrite in very low pH (highly acid) conditions has created the required environment for formation of antlerite, which occurs as a post-mining mineral in association with brochantite. Crystals are only visible under the scanning electron microscope (MW).
Alteration
antlerite and water to brochantite and and sulphuric acid
4Cu3SO4(OH)4(s) + 2H2O(l) → 3Cu4SO4(OH)6(s) + H2SO4(aq)
(JRS 18.12, 13)
chalcanthite (s) and Cu2+ (aq) to antlerite (s), H+ (aq) and H2O(l)
CuSO4.5H2 + 2Cu2+ ⇌ Cu3SO4(OH)4 + 4H+ + H2O
(MM 52.683)
The Activity-pH diagram below was calculated at 298.2 K for some arsenates and sulphates for constant activity (roughly equivalent to concentration) of H2AsO4- in solution, over a range of values of pH and of SO42- activity (MM 52.689).
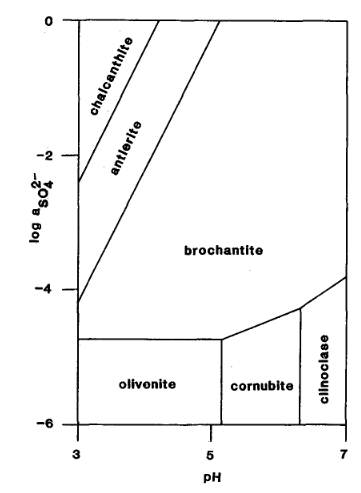
The mineral formulae are:
chalcanthite: Cu(SO4).5H2O
antlerite: Cu2+3(SO4)(OH)4
brochantite: Cu4(SO4)(OH)6
olivenite: Cu2(AsO4)(OH)
cornubite: Cu5(AsO4)2(OH)4
clinoclase: Cu3(AsO4)(OH)3
Back to Minerals