Chalcanthite
chalcanthite
antlerite
brochantite
chalcopyrite
Images
Formula: Cu(SO4).5H2O
Hydrated sulphate, chalcanthite group
Crystal System: Triclinic
Specific gravity: 2.286 measured, 2.282 calculated
Hardness: 2½
Streak: Blue
Colour: Blue
Solubility: Readily soluble in water, hydrochloric, sulphuric and nitric acid
Common impurities: Fe,Mg,Co
Environments:
Hydrothermal environments
Chalcanthite is a secondary mineral that is found in the oxidation
zone of copper deposits. It forms only in conditions of low pH (very acid) and high
sulphate concentration, which occur only in exceptionally arid conditions; normally copper and sulphate ions remain
in solution. Chalcanthite is formed by the oxidation of chalcopyrite and other
copper sulphates. It is often found as a post-mining deposit on mine walls,
where it crystallises from mine waters. When the carbonate concentration is similar to the atmospheric value, at
successively higher values of pH (more alkaline conditions), chalcanthite is replaced by
antlerite then brochantite then
malachite
(JRS 18.12, 13).
Alteration
chalcanthite (s) and Cu2+ (aq) to antlerite (s),
H+ (aq) and H2O(l)
CuSO4.5H2O + 2Cu2+ ⇌ Cu3SO4(OH)4 + 4H+
+ H2O
(MM 52.683)
The Activity-pH diagram below was calculated at 298.2 K for some arsenates and
sulphates for constant activity (roughly equivalent to concentration) of H2AsO4- in solution, over a
range of values of pH and of SO42- activity
(MM 52.689).
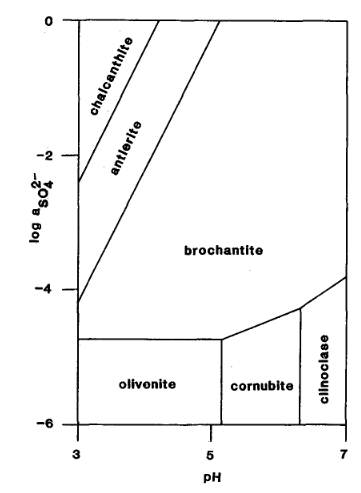
The mineral formulae are:
chalcanthite: Cu(SO4).5H2O
antlerite: Cu2+3(SO4)(OH)4
brochantite: Cu4(SO4)(OH)6
olivenite: Cu2(AsO4)(OH)
cornubite: Cu5(AsO4)2(OH)4
clinoclase: Cu3(AsO4)(OH)3
Back to Minerals
