Localities
At Corocoro district, Pacajes province, La Paz department, Bolivia, copper pseudomorphs after aragonite cyclic twins have been found (KL p119).
Copper from Corocoro - Image
At the Chengmenshan Mine, Jiujiang County, Jiujiang, Jiangxi, China, dentritic native copper occurs on a white matrix (AESS).
Copper from Chengmenshan - Image
At the Rubtsovskiy mine, Russia, copper pseudomorphs after cuprite have been found (KL p121, R&M 95.3.275).
At Tsumeb, Namibia, mottramite pseudomorphs after copper have been found (KL p201).
Copper is the most abundant native element in the Tsumeb orebody. It is of secondary origin and is found in all three oxidation zones, but especially in the upper levels, occasionally in cuboctahedral crystals and spear-shaped spinel-law twins to 5 cm. It forms dendritic aggregates and nail-like crystals and crystalline masses up to 20 cm, associated with cuprite, dolomite, malachite, cerussite and calcite
Copper from Tsumeb - Image
At the Bardon Hill quarry, Coalville, Leicestershire, England, UK, native copper is found associated with cuprite and altering to malachite (RES p194).
At the New Cliffe quarry, Stanton under Barton, Leicestershire, England, UK, native copper is found associated with cuprite and altering to malachite (RES p194).
Copper from the New Cliffe Quarry - Image
At the Old Dominion mine, Gila county, Arizona, USA, copper pseudomorphs after cuprite have been found (R&M 94.2.169).
Copper from the Old Dominion Mine - Image
At the Magma mine, Pioneer District, Pinal county, Arizona, USA, native copper has been found sporadically throughout the mine, usually on a calcite matrix, although the metal is more commonly associated with the oxidised zones of copper deposits (R&M 95.1.84).
Copper from the Magma Mine - Image
At the Quincy Mine, Quincy Township, Houghton County, Michigan, USA, a spectacular specimenn of copper with calcite and epidote has been found (Mindat photo).
Copper from the Quincy Mine - Image
At many localities in Keweenaw county, Michigan, USA, excellent specimens of crystallised copper have been found, as well as copper banded agates. Specimens of copper have been found on prehnite, encrusted with silver or cuprite or enclosed by calcite (R&M 97.4.354-363).
The Central Mine, Central, Keweenaw county, Michigan, USA, initially targeted a series of sub-parallel mineralised fissure veins where the most copper-rich portion of the vein was close to the base of the main greenstone flow.
The sheer number of fine and highly varied copper specimens the Central mine has produced makes it a premier locality for the species worldwide. Copper crystals from the Central mine are predominantly tetrahexahedrons, sometimes modified by the cube or dodecahedron, and commonly form arborescent clusters. Numerous superb Central mine copper specimens exist in public and private collections which feature large (2 to 5 or more cm) individual, equant crystals in aggregates or as floaters.
Classic herringbone groups of elongated spinel-twinned copper crystals are typical of the Central mine.
Crystallised copper here is commonly associated with silver, calcite, quartz, prehnite, adularian orthoclase, pumpellyite, datolite and more rarely with actinolite, hematite and chalcocite (MinRec 54.1.53-81).
Copper from the Central Mine - Image
At the Copper Falls Mine, Copper Falls, Keweenaw county, Michigan, USA, mineralisation occurs primarily in hydrothermal veins cutting preexisting lavas and as amygdules in the Ashbed flow.
Native copper is the primary ore species. It occurs mainly as amygdule fillings in the Ashbed flow and as masses and disseminated material in veins. Masses have exceeded 70 tons. Crystals of copper are restricted to the various veins and show a wide variety of habits. Thin, epitaxial overgrowths of copper on a variety of other minerals have been noticed (MinRec 54.1.105-107).
Copper from the Copper Falls Mine - Image
The Cliff Mine, Phoenix, Keweenaw county, Michigan, USA, is situated at the base of a roughly 70-metre basalt cliff. A curious feature of the impressive thickness of the greenstone flow here is that it contains zones of “pegmatoid”: areas where slow cooling in the core of the lava flow allowed for large feldspar crystals exceeding 1 cm to grow. Such features are normally only observed in intrusive igneous rocks and are almost unheard of in basalt flows.
The Cliff mine primarily exploited rich copper mineralisation in the Cliff fissure (vein). Although mineralised with copper to some extent along its entire length, the part of the vein just below the greenstone flow carried the richest copper mineralisation by far. A significant amount of the copper recovered at the Cliff mine came from amygdaloids in the tops of 13 basalt flows which were cut by the Cliff vein. The discovery and mining of this vein proved that the veins were the source of the large masses of float copper that were already well known, and proved that the primary ore mineral in the district was native copper, not sulphides, as had been suspected earlier.
As the primary ore mineral at the Cliff mine, copper, as masses up to many tons or as finely disseminated grains in veins and amygdaloids near the veins, was abundant. Crystal habits for copper from the Cliff mine include the cube, octahedron and tetrahexahedron, although most specimens show crystals with complex intergrowths, including twins of multiple habits. Arborescent crystal groups and wire copper are also fairly common. Coatings of the copper oxides cuprite and tenorite, giving red and black colours respectively, are often observed in specimens from the Cliff mine (MinRec 54.1.25-49).
Copper from the Cliff Mine - Image
At Big Sucker Creek, St. Louis County, Minnesota, USA, copper has been found with prehnite (Mindat photo).
Copper from Big Sucker Creek - Image
At the Chimney Rock Quarry, Bridgewater Township, Somerset County, New Jersey, USA, specimens of copper have been found, some of which have partial coatings of very bright blue-green chrysocolla, and probably the chrysocolla has other blue secondary species mixed in with it as well. What’s more, some of the specimens are spotted lightly with microcrystals, to 2 mm, of native silver. (MinRec 55.3.353-355).
Copper from the Chimney Rock Quarry - Image
At Georgetown, Grant county, New Mexico, USA, copper pseudomorphs after azurite have been found (KL p120).
Copper from Georgetown - Image
At the Kabwe mine, Central Province, Zambia, small amounts of native copper have been found associated with malachite, cuprite and chalcocite (R&M 94.2.124).
Copper from Kabwe - Image
At the Mufulira Mine, Mufulira, Mufulira District, Copperbelt Province, Zambia, supergene native copper and cuprite were common in the orebody. The copper is coarsely disseminated in the quartzite, where it occurs as thin sheets on bedding planes and joint surfaces. On the upper levels of the mine, massive copper lumps weighing 45 kilograms were encountered in some of the voids. The Mufulira mine is justly famous for its native copper specimens, many displaying spinel-law twinned crystals. (MinRec 55.4.461-465).
Copper from Mufulira - Image
Alteration
chalcocite and oxygen to native copper and sulphate ions
Cu2S(s) + 2O2(g) → Cu(s) + Cu2+(aq) + SO42-(aq)
If acidic copper sulphate solutions pass through the oxidation zone to below the water table, conditions usually change to reducing and the dissolved copper ions react with sulphide ions (S2-) to form copper sulphides such as chalcocite. If the water table falls, allowing the chalcocite to be exposed to the oxidation zone, then native copper can form according to the above reaction (JRS 18.14).
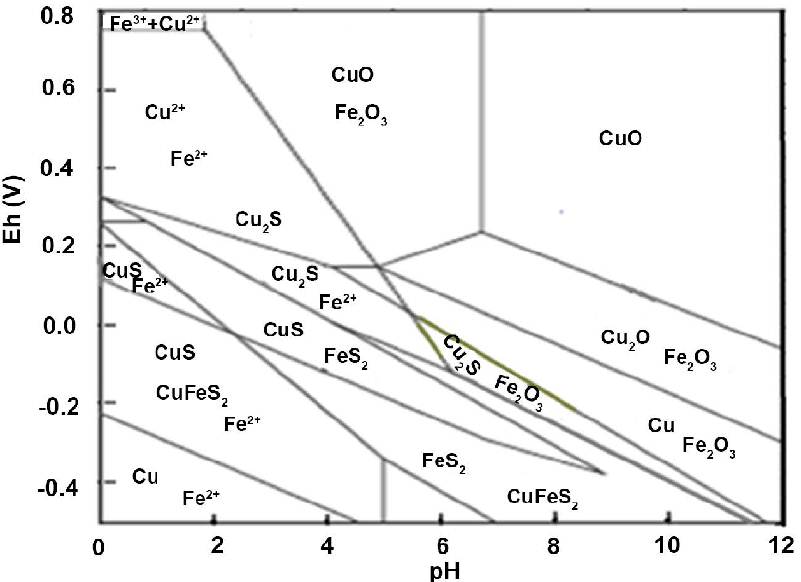
The diagram below is a Pourbaix diagram for Cu-Fe-S-H2O (IJNM 07(02).9.23). It shows the relationship between copper Cu, chalcopyrite CuFeS2, tenorite CuO, covellite CuS, cuprite Cu2O, chalcocite Cu2S, pyrite FeS2 and hematite Fe2O3.
Back to Minerals