2Cu2+ + HS- + 2e- → Cu2S + H+
Chalcocite has a stability range at any pH (acid or alkaline) in a relatively reducing environment.
Localities
The Two Mile and Three Mile deposits, Paddy's River, Paddys River District, Australian Capital Territory, Australia, are skarn deposits at the contact between granodiorite and volcanic rocks. Chalcocite occurs both as a primary sulphide and as a supergene mineral replacing chalcopyrite and sphalerite along fractures and grain boundaries. Primary chalcocite has been found as intergrowths with bornite (AJM 22.1.36).
At the Mount Kelly deposit, Gunpowder District, Queensland, Australia, the deposit has been mined for oxide and supergene copper ores, predominantly malachite, azurite and chrysocolla. The ores overlie primary zone mineralisation consisting of quartz-dolomite-sulphide veins hosted in dolomite-bearing siltstone and graphitic schist.
Chalcocite was found as a dull, blue-grey supergene mineral replacing chalcopyrite in the early stages of oxidation (AJM 22.1.19).
At the At the Mount Lyell mines, Queenstown district, West Coast municipality, Tasmania, Australia, chalcocite is common in many of the sulphide ore deposits, sometimes disseminated with bornite in schist, and sometimes as blocks weighing many kilograms (AJM 21.2.22).
At Saint-Pierre-de-Broughton, Les Appalaches RCM, Chaudière-Appalaches, Quebec, Canada, chalcocite occurs in the talc-carbonate rocks in solid sulphide veins and masses intergrown with the more common bornite and chalcopyrite. It also occurs in massive quartz veins and lenses, as irregular black metallic patches in bornite and associated with chalcopyrite (R&M 85.6.502).
At Charcas, Charcas Municipality, San Luis Potosí, Mexico, the primary minerals are sphalerite, galena, chalcopyrite, bornite, tetrahedrite, arsenopyrite, pyrite and silver minerals such as jalpaite, diaphorite and acanthite. In the host rock, as metamorphic or alteration minerals, danburite, datolite, hedenbergite, epidote, chlorite, andradite, actinolite and wollastonite have been reported.
Quartz, calcite and danburite crystallised during the entire life of the systems, throughout the intrusive emplacement, metamorphism, and mineralising events. With depth, both sphalerite and galena decrease while chalcopyrite increases.
Secondary sulphides formed include bornite, covellite, digenite and chalcocite. Native silver, native gold, hematite and goethite were deposited after the sulphides (Minrec 55.6.727-728).
Massive chalcocite has been reported as widely distributed across the district, but specimens exhibiting well formed crystals are scarce. Chalcocite generally occurs in microscopic form as a replacement of digenite. When encountered the crystals are lustrous or dull and black, or black with blue reflections. They have a flat hexagonal shape and may show twinning. Chalcocite crystals reaching 1.5 cm on edge are generally of older vintages, although in 2019 five miniature-size specimens were collected at the Rey y Reina mine (Minrec 55.6.743).
At Tsumeb, Namibia, chalcocite was quite common, associated with native silver (R&M 93.6.542). Pseudomorphs of chalcocite after galena have been found here (KL p128).
Chalcocite was one of the most important of the sulphide ore minerals at Tsumeb, typically occurring as dark lead-grey massive ore and very rarely as thick to tabular pseudohexagonal crystals. The finest crystals, up to 4 cm, were found in the third oxidation zone, where they were associated with stolzite, pink smithsonite, siderite and scheelite (Minrec 55.6 supplement p87).
Chalcocite from Tsumeb - Image
At the M'Passa Mine, Mindouli District, Pool Department, Republic of the Congo, chalcocite crystals occur with associated pyrite (Dr Marco Tam Shing Yau, The Mineralogy Society of Hong Kong Newsletter 19.8).
Chalcocite from the M'Passa Mine - Image
At the Nababeep West Mine, Nababeep tungsten mines, Nababeep, Okiep Copper District, Namakwa District Municipality, Northern Cape, South Africa, one of the finest specimens of chalcocite from South Africa has been found (Mindat photo).
Chalcocite from the Nababeep West Mine - Image
At the Mariquita Mine (Sultana Mine), Usagre, Badajoz, Extremadura, Spain, chalcocite is usually found in massive form, but occasionally it occurs in small vugs as groups of platy hexagonal crystals individually reaching 2 mm in size, with quartz and baryte (MinRec 55.4.496).
Chalcocite from the Mariquita Mine - Image
At Geevor Mine, Pendeen, St Just, Cornwall, England, UK, chalcocite has been found as rare, pseudohexagonal prismatic crystals, rather than the more common massive material (AESS).
Chalcocite from Geevor - Image
At the Magma mine, Pioneer District, Pinal county, Arizona, USA, chalcocite has been found on a hematite matrix (R&M 95.1.83-84).
Chalcocite from the Magma Mine - Image
At the Copper Falls Mine, Copper Falls, Keweenaw county, Michigan, USA, mineralisation occurs primarily in hydrothermal veins cutting preexisting lavas and as amygdules in the Ashbed flow.
Chalcocite is very rare at the Copper Falls mine. A single significant specimen, with long, striated crystals to 2 cm, is in the A E Seaman Mineral Museum (MinRec 54.1.105).
At the Leonard mine, Montana, USA, chalcocite pseudomorphs after covellite have been found (KL p127).
Chalcocite from the Leonard Mine - Image
At the Mufulira Mine, Mufulira, Mufulira District, Copperbelt Province, Zambia, most chalcocite occurs as dense disseminations on bedding planes in the quartzite host rock, but it rarely forms sizeable masses. Crystals of chalcocite, although often occurring as well-formed tabular, pseudohexagonal prisms, are small (a few millimeters), rare, and generally found in vuggy veins with bornite and chalcopyrite in the eastern sections of the mine (MinRec 55.4.456).
Chalcocite from the Mufulira Mine - Image
Alteration
Oxidation of pyrite forms ferrous (divalent) sulphate and sulphuric acid:
pyrite + oxygen + water → ferrous sulphate + sulphuric acid
FeS2 + 7O + H2O → FeSO4 + H2SO4
The ferrous (divalent) sulphate readily oxidizes to ferric (trivalent) sulphate and ferric hydroxide:
ferrous sulphate + oxygen + water → ferric sulphate + ferric hydroxide
6FeSO4 + 3O + 3H2O → 2Fe2(SO4)3 + 2Fe(OH)3
chalcocite to covellite
Ferric sulfate is a strong oxidizing agent; covellite is formed from chalcocite by the reaction below.
chalcocite and ferric sulphate to copper sulphate, ferrous sulphate and covellite
Cu2S + Fe2(SO4)3 → CuSO4 + 2FeSO4 + CuS
(AMU b3-3.7)
chalcocite to cuprite
If chalcocite is exposed to the oxidation zone, then conditions for the formation of cuprite and native copper can occur readily.
chalcocite + oxygen + water → cuprite + sulphuric acid
Cu2S(solid) + 2O2(gaseous) + H2O(liquid) → Cu2O(solid) + H2SO4(aqueous)
(JRS 18.14)
chalcocite to native copper
chalcocite + oxygen → copper + cupric sulphate
Cu2S(solid) + 2O2(gaseous) → Cu(solid) + Cu2+SO4(aqueous)
(JRS 18.14)
chalcopyrite and chalcocite to bornite
CuFe3+S2 + 2Cu2S = Cu5FeS4
pyrite to chalcocite
Because chalcocite is less soluble than pyrite, supergene chalcocite may form below the zone of oxidation when dissolved copper ions Cu2+ replace ferrous ions Fe2+ from pyrite.
Cu2+ + pyrite + H2O to chalcocite + Fe2+ + (SO4)2- + H+
14Cu2+ + 5FeS2 + 12H2O → 7Cu2S + 5Fe2+ + 3(SO4)2- +24H+
(KB p527)
skinnerite to chalcocite, antimony and sulphur
2Cu3SbS3 → 3Cu2S + 2Sb + 3/2S2
(CM 28.725-738)
skinnerite and sphalerite = Zn-tetrahedrite and chalcocite
4Cu3SbS3 + 2ZnS → Cu10Zn2Sb4S13 + Cu2S
(CM 28.725-738)
Zn-tetrahedrite to chalcocite, antimony, sphalerite and sulphur
Cu10Zn2Sb4S13 → 5Cu2S + 4Sb + 2ZnS + 3S2
(CM 28.725-738)
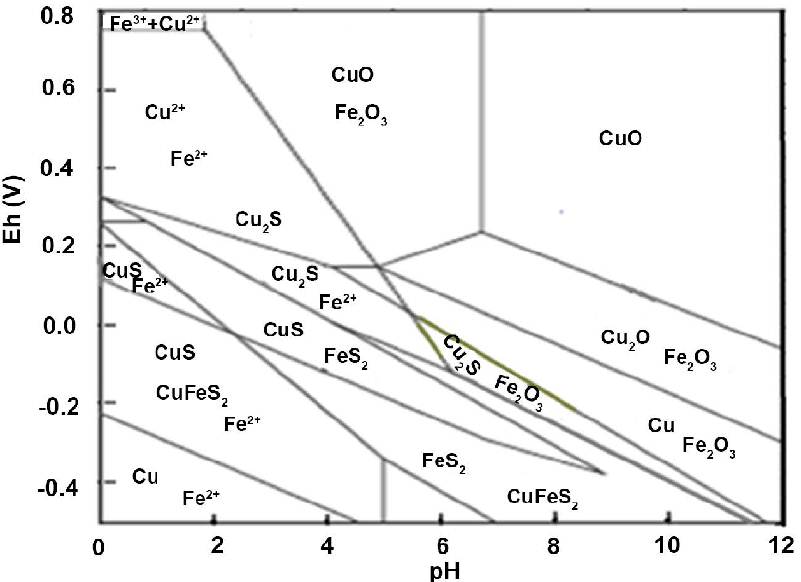
The diagram below is a Pourbaix diagram for Cu-Fe-S-H2O (IJNM 07(02).9.23). It shows the relationship between copper Cu, chalcopyrite CuFeS2, tenorite CuO, covellite CuS, cuprite Cu2O, chalcocite Cu2S, pyrite FeS2 and hematite Fe2O3.
Back to Minerals