Antimony
antimony
silver
arsenic
stibnite
Images
Formula: Sb
Native element, metalloid, arsenic group
Forms a series with arsenic
Specific gravity: 6.61 to 6.71
Hardness: 3 to 3½
Streak: Grey
Colour: Tin-white
Antimony has the unusual property that, like water, it expands as it freezes. Four other elements expand when they freeze, namely
silicon, bismuth, gallium and germanium (ChC).
Melting point: 630.79oC
Boiling point: 1587oC
Common impurities: As
Environments:
Hydrothermal environments
Abundance is 0.2 parts per million by mass, 0.03 parts per million by moles in the Earth's crust, and 950 parts per billion by mass, 10
parts per trillion by moles in the Solar System (ChC)
Antimony is seldom found in the native state, but it does occur in
antimony-silver
hydrothermal veins with
silver, antimony and
arsenic minerals
(Webmin, Dana, HOM).
Associated minerals include silver,
stibnite, stibarsen,
sphalerite,
pyrite, galena and
quartz
(HOM, Dana, Mindat).
Localities
At Wet Swine Gill, Coombe Height, Caldbeck, Allerdale, Cumbria, England, UK, veins of native antimony in
association with coatings of yellow bindheimite have been found
(AESS).
Antimony from Wet Swine Gill - Image
At the Driggith mine, Caldbeck Fells, Cumbria, England, UK, minute grains of antimony are associated with
inclusions of
bournonite in galena
(C&S).
Alteration
skinnerite to chalcocite,
antimony and sulphur
2Cu3SbS3 → 3Cu2S + 2Sb + 3/2S2
(CM 28.725-738)
Zn-tetrahedrite to chalcocite,
antimony, sphalerite and
sulphur
Cu10Zn2Sb4S13 → 5Cu2S + 4Sb + 2ZnS + 3S2
(CM 28.725-738)
Zn-tetrahedrite to skinnerite,
antimony, sphalerite and
sulphur
3Cu10Zn2Sb4S13 → 10Cu3SbS3 + 2Sb + 6ZnS + 3/2S2
(CM 28.725-738)
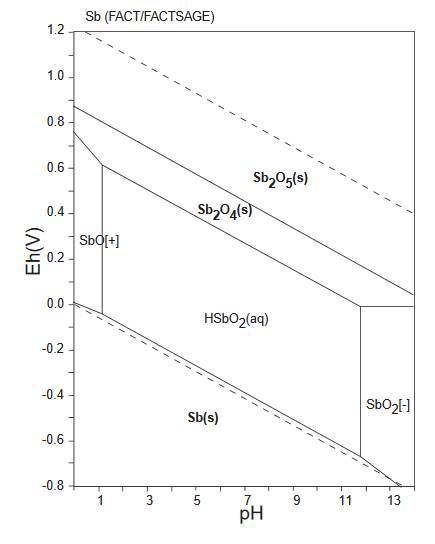
The diagram below is a Pourbaix diagram for antimony (GSJ).
It shows the relationship between antimony Sb and
cervantite/clinocervantite
Sb2O4.
The commonest ore of antimony is stibnite
Antimony-bearing minerals include:
Alloys - an alloy is a substance that combines more than one metal or mixes a metal with other non-metallic
elements
Arsenides - the arsenide anion is As3-
Antimonides - the antimonide anion is Sb3-
Sulphides - the sulphide anion is S2-
Selenides - the selenide anion is Se2-
Tellurides - the telluride anion is Te2-
Sulphosalt - AmBnXp: where A is a metal , B usually a semi-metal and X
is sulphur, selenium or tellurium
Oxides - the oxide anion is O2-
Hydroxides - the hydroxide anion is (OH)-
Chlorides - the chloride anion is Cl-
Sulphates - the sulphate anion is (SO4)2-
Tantalates - salts of tantalic acid (Ta5+O3)-
Tellurites - the tellurite anion is (Te4+O3)2-
Arsenates - the arsenate anion is (As5+O4)3-
Antimonates - the antimonate anion is (Sb5+O3)-
Antimonites - the antimonite anion is (Sb3+O3)3-
Back to Minerals
